

Paint is used to decorate, protect and prolong the life of natural and synthetic materials, and acts as a barrier against environmental conditions.Paints may be broadly classified into Decorative paints, applied on site to decorate and protect buildings and other objects, and Industrial coatings which are applied in factories to finish manufactured goods such as cars.
The constituents of paint
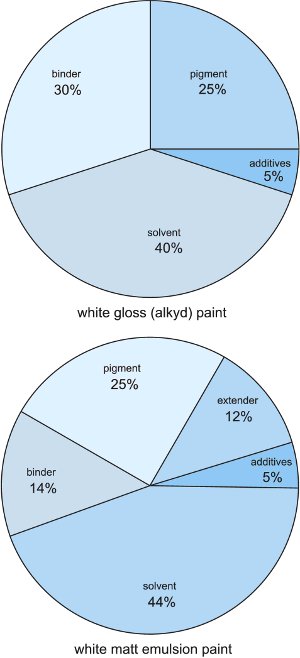
Paints contain:
The binder (resin) and solvent together are sometimes known as the vehicle. The binder may be dissolved as a solution or carried as a dispersion of microscopically small particles in a liquid.
Depending on the type of paint and intended use, additives may include:
Paints are formulated according to their proposed use - primer, undercoat, special finishes (matt, gloss, heat resistance, anti-corrosion, abrasion resistance). The pigment powder is broken down into individual particles which are coated by and dispersed in the binder (resin) - known as 'wetting out'. Solvent is then added to give the required consistency. Each batch of ingredients is thoroughly mixed in large, stirred containers with the required additives (Figure 1). Amounts ranging up to 40 000 dm3 of paint may be made in a single batch.
This unit discusses the most commonly used binders followed by the pigments.
The three most important binders (resins) used in modern paints are:
The binder in many emulsion paints is based on homopolymers or co-polymers of ethenyl ethanoate (vinyl acetate) and a propenoate (acrylic) ester. Ethenyl ethanoate is manufactured by passing a mixture of ethanoic acid vapour, ethene and oxygen over heated palladium(ll) and copper(ll) chlorides:

Ethenyl ethanoate and an acrylic ester (for example, methyl 2-methylpropenoate) are then co-polymerized to form a random array, in which these groups link into a linear chain:
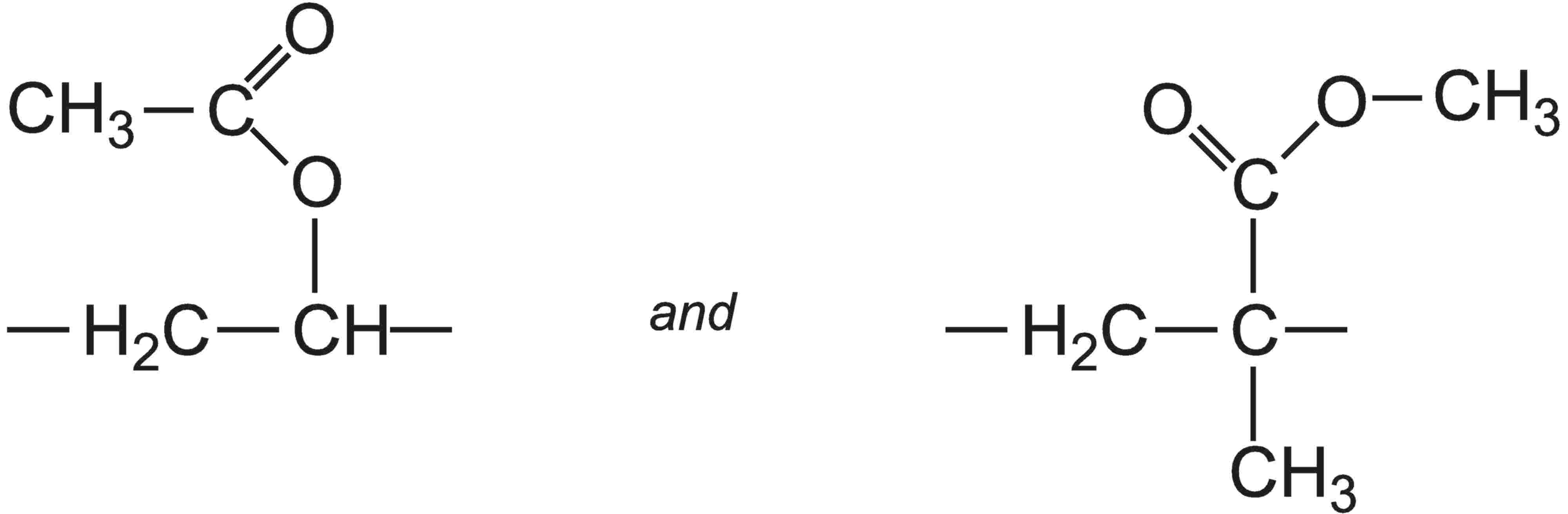
Other acrylic esters used as co-monomers with ethenyl ethanoate are ethyl propenoate, butyl propenoates, or a co-polymer of butyl propenoate and methyl 2-methylpropenoate.
The polymers used in these paints are carried in water (water-borne emulsion paints) which as described above are much better for the environment than paints in which the binders are in organic solvents.
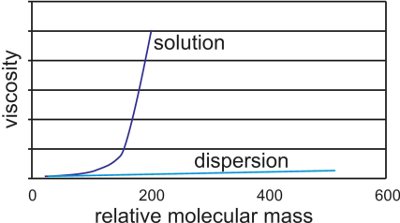
Emulsion paints are so-called as they are made by a process known as emulsion polymerization, in which the liquid monomers to be polymerized are first dispersed in water, as an emulsion. The polymers produced by this process typically have relative molecular masses of 500 000 - 1 000 000. As such they are useful only as dispersions since they would be extremely viscous if they were carried in solution and this would make them unusable.
Acrylic resins may also be used in industrial paints, either as water-borne emulsion paints or as solvent-borne paints. Solvent-borne industrial paints can have a tough protective finish and are widely used in industry as topcoats, for example on car bodies. The paint frequently comes as two components which are mixed together just before use: the main paint portion typically consists of an acrylic resin produced by the polymerization of a propenoate ester formed from a polyhydric alcohol (diols and triols). The resulting polyester has numerous hydroxyl groups (-OH) pendant from the polymer backbone. The hydroxyl groups react with the other compound often consisting of a polymeric isocyanate such as a trimer of 1,6-diisocyanatohexane (hexamethylene diisocyanate):
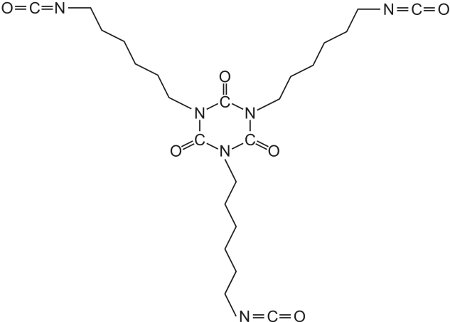
Such a compound is known as a crosslinker for it produces, on reaction with the resin, a three-dimensional structure similar to the polyurethane formed from a polyol and an isocyanate.
When these two components are mixed together, a chemical reaction takes place between the hydroxyl groups on the polymer (acrylic resin) and the isocyanate groups on the cross linker:
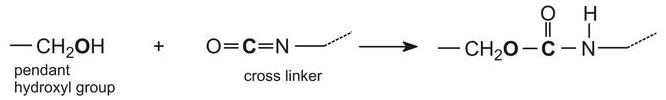
This reaction proceeds relatively slowly at room temperature, allowing enough time for the paint to be applied, after which the solvent thinner evaporates and the painted item is placed in an oven to accelerate the chemical reaction. This greatly increases the molecular mass of the polymer causing it to become a three-dimensional molecule and form a hard film, resistant to chemicals.
Office : 98/11, ONKAR NAGER-B, TRI NAGER,DELHI-110035
Phone no: 27383849, +91 9873597319, +91 9811013802
Email: Unichem_123@Yahoo.Com, Unichem_123@Rediffmail.Com